Tosoh
Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based Chromatography Technique
Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based Chromatography Technique
Description
Most clinically approved large biotherapeutics are monoclonal antibodies (mAbs),
primarily belonging to immunoglobulin G subclass-1 (IgG1) and, to a lesser extent,
IgG2 and IgG4. Glycosylation is the main source of post-translational heterogeneity of
mAbs, impacting their drug therapeutic mechanism of action (MOA). Glycosylation
is also one of the critical factors in drug product solubility, kinetics, stability and
efficacy. Thus, monitoring glycan critical quality attributes (CQAs) is an essential part
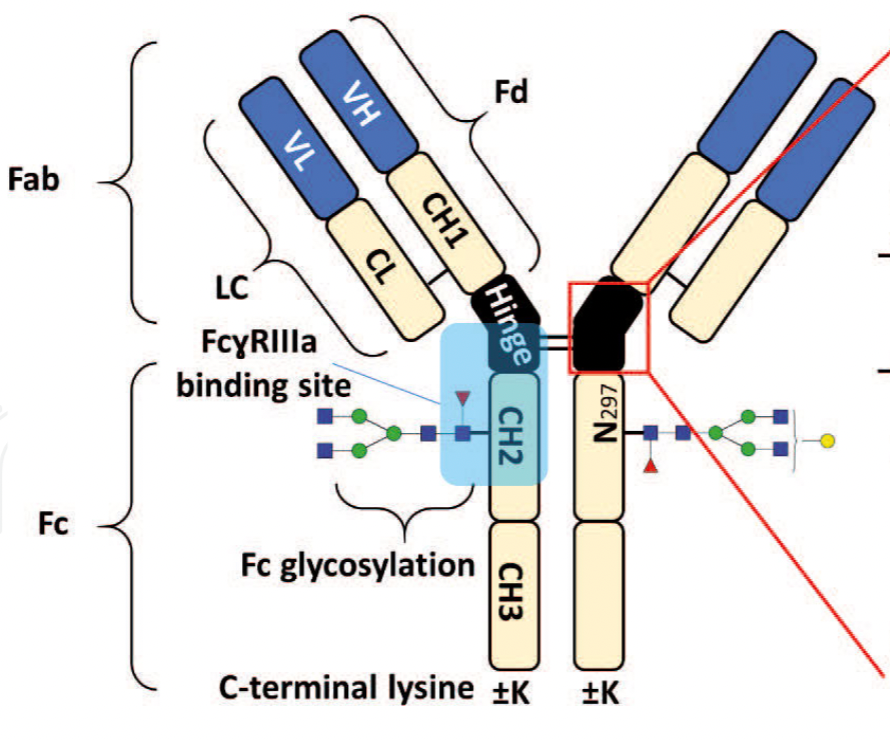
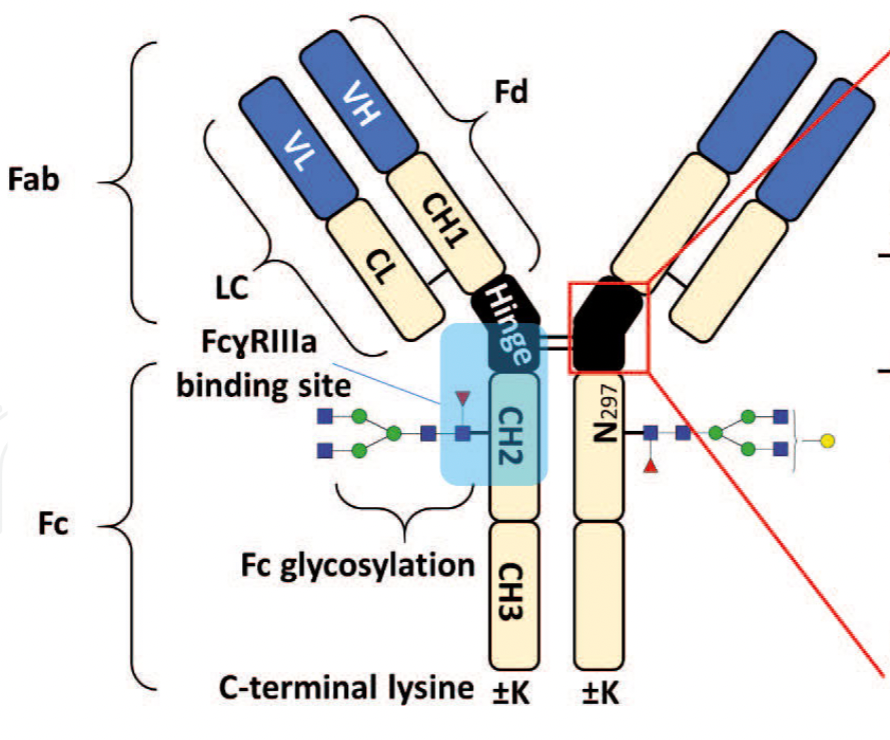
of any biopharmaceutical development. The binding affinity of an IgG to its cellular Fc
receptor (FcR) depends on both its IgG subclass and Fc domain glycosylation pattern.
Since composition of the N-glycans also correlates to the Antibody-Dependent Cellular
Cytotoxicity (ADCC), the glycosylation pattern needs to be monitored for consis-
tency in potency and efficacy. This applies for the original mAb biologics as well as
biosimilars. In this chapter, we present a truly novel way to assess the variances in mAb
glycoforms using FcγRIIIa-based affinity chromatography. First, a brief overview of the
Fc receptor function is presented. Then, the principle of FcR-based affinity chromatog-
raphy is explained including how this column’s potential to analyze a variety of mAbs
according to their N-glycan content is highly selective and robust. Finally, we provide
examples of the FcR column’s potential to improve analytical characterization of mAbs
with practical applications such as effective cell line screening, monitoring of glycoengineering, process development and process control in manufacturing.
Keywords: FcR, glycoform, N-glycan, monoclonal antibody, mAb, biosimilar,
affinity chromatography, antibody-dependent cellular cytotoxicity, ADCC
1. Introduction
Affinity chromatography is a popular method for the purification of biomolecules. The purification involves interaction of the biomolecule with a ligand covalently immobilized to a solid stationary phase. Elution occurs as a function of binding strength of the biomolecule to the stationary phase, tighter the binding later the elution time in a linear gradient. Due to high selectivity and fast separation, affinity chromatography is regarded as the most widely used purification method for capture step in biopharmaceutical industry. Different types of affinity Monoclonal Antibodies 2 chromatography ligands are available as resins and pre-packed columns for purification at various scales. Similarly, different types of U/HPLC affinity chromatography columns are available for analytical characterization and quality control. This chapter focuses on a recently introduced novel FcR-based affinity column, TSKgel- FcR-IIIA-NPR, that enables chromatographic characterization of mAbs based on their N-glycan content attached to a highly conserved Asn-297 in Fc region.